Disulfiram | An Innovative Treatment for Persistent Lyme
Disulfiram may help arm you in your fight against persistent Lyme.
A chemical variation of disulfiram was first used in the early 1900’s for the sulfur vulcanization of rubber. In the 1930’s a rubber factory doctor noticed workers having negative effects from alcohol consumption after exposure to disulfiram. Around the same time disulfiram was being tested for the treatment of various parasitic infections. In the 1940’s chemists at a Danish drug company accidently contaminated a batch of disulfiram and it was found to have better drug properties. It was then patented as Antabuse. It was approved in 1951 by the FDA for the treatment of alcohol use disorder.
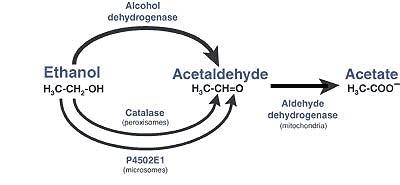
At that time it was also discovered how alcohol was metabolized. It is a two step detoxification process that occurs mostly in the liver, but also in the brain, pancreas, and stomach. Alcohol is converted to the TOXIC intermediate ACETALDEHYDE which is then quickly is converted to acetate by the enzyme ALDEHYDE DEHYDROGENASE. The toxic intermediate acetaldehyde is what can cause symptoms of a “hangover” such as headache, nausea, and vomiting.
Disulfiram works by BLOCKING the enzyme ALDEHYDE DEHYDROGENASE which leads to the accumulation of acetaldehyde. Upon immediate ingestion of alcohol this build up of acetaldehyde will lead to symptoms of a severe “hangover”.
After early use of disulfiram as a possible anti-parasitic agent, evidence had been gathering of its effectiveness against parasites such as giradia and malaria. Current research investigating possible antibacterial properties have revealed effectiveness against many bacterial species including mycobacteria and gram positive bacteria such as staph, strep, and bacillus. Possible mechanisms of antimicrobial activity include disruption of bacterial replication by the sulfur in disulfiram.
In 2016, researchers at Stanford University looked at over 4,000 different compounds that could potentially have high antibacterial activity against Borrelia burgdorferi, the bacteria that causes Lyme disease.2 They found about 150 compounds that had greater than 90% killing activity, 101 of which were already FDA approved for other conditions. They chose to closely investigate the top 20 compounds, one of which was disulfiram.
Over recent years, disulfiram is now gaining popularity as a possible tool in the arsenal for the treatment of lyme disease symptoms that persist after initial treatment. There is even an ongoing clinical trial through Columbia University investigating the effectiveness of disulfiram for persistent symptoms after initial infection.3
At the Stram Center disulfiram has been used for a select number of patients who experience persistent symptoms after inital Lyme infection. Our clinical experience has been mixed. While some have dramatic symptomatic improvement, others report a much worsening of symptoms. It is sometimes difficult to determine whether the worsening is from the treatment of the infection or possible drug side effect. Disulfiram side effects are many, and have included mental emotional instability, extreme fatigue, tingling and numbness, and headaches. Dosing is by body weight and is started low and is slowly increased over time to minimize side effects. We also recommend supplemental protocols that can lessen side effects.
The treatment time is typically 3 months but can be up to 6 months. During this time there can be no consumption of alcohol or alcohol based products, foods, and avoidance of topical products that contain alcohol as these can worsen side effects.
Disulfiram can be a potential promising therapy for persistent symptoms post lyme infection.
To see if this therapy could be beneficial for you, please contact us at the Stram Center.
1) Diagram from:
Alcohol Research & Health, Vol. 29, No. 4, 2006
National Institute on Alcohol Abuse and Alcoholism Publications Distribution Center
2) Drug Des Devel Ther. 2016 Apr 1;10:1307-22. doi: 10.2147/DDDT.S101486. eCollection 2016.
Identification of new drug candidates against Borrelia burgdorferi using high-throughput screening.
Pothineni VR1, Wagh D1, Babar MM1, Inayathullah M1, Solow-Cordero D2, Kim KM1, Samineni AV1, Parekh MB1, Tayebi L3, Rajadas J1.
3) https://recruit.cumc.columbia.edu/clinical_trial/1661