Analysis of Lyme: Attachment, Transmission, Stages, Challenges, and Treatment

In this analysis of the current literature and research on Lyme and tick borne illnesses by Dr. Ronald Stram and medical assistant, Ahn Vo. Starting with an overview of its history; tick attachment time and rate of transmission; stages of infection and related symptoms; challenges in Lyme and testing; and antibiotics versus combination therapy. This throughout analysis contains a wealth of information for those who want to expand their knowledge about Lyme and it's complexity.
Lyme Disease and Tick-borne Illness
According to the CDC, Lyme disease (LD) is the most common vector-borne disease reported in the United States [1]. Typically, patients that are diagnosed early and treated accordingly are returned to health. Tick endemic areas of the United States not only have Lyme to contend with but other tick-borne illnesses such as Rocky Mountain spotted fever, Babesia, Bartonella, ehrlichiosis, and more [2]. Since ticks are such excellent vectors of many diseases, pathogens, and viruses, anyone bitten by a tick should test the tick they pull off. However, regardless of the tick testing being negative any new onset of symptoms following the bite should be addressed.
Tick Attachment Time and Rate of Transmission
Removal of the tick, even prompt removal does not guarantee prevention of disease. Many guidelines state that the risk of infection by LD (and by extension tick-borne illness) increases the longer the period the tick is attached. These guidelines state that transmission of infection occurs when the tick is attached >24 hours even up to 72 hours [3]. A study in 1987 by Piesman et al., looked at the rate of infection of hamsters and mice based on length of time of tick feeding. This study demonstrated a 7% infection rate of the rodents at <24 hours, 33% at <48 hours and 93% at <72 hours [4]. According to a review by Cook, the confidence interval for transmission <24 hours is ±13.37% and for <48 hours is ±24.63%. Respectively the probability for transmission can be as high as 20.37% within 24 hours and 57.63% within 48 hours [3]. A second study by Piesman in 1993 demonstrated similar data, 7% infection rate at <36 hours, 25% at 42 hours and 75% in 48 hours [5]. Another study in 1993 by Shih and Spielman, also concluded that infection typically happened when the tick fed for 2 days. However, they also found in another experiment that ticks that had partially fed could re-attach to a new host and in 24 hours there was a 83% infection rate [6]. This has significance to patients with companion animals.
Unfortunately, these studies do not confirm claims of the prevention of infection with removal of the tick within 24-72 hours of attachment. Once ticks are attached, they release their saliva which is a mixture of antihistamines, cytokine inhibitors and anticoagulants through the course of their meal. The explanation that was proposed for the alleged time delay between attachment and transmission of infection by the tick was due antigenic changes the B.burgdorferispirochete would need to undergo to infect humans and travel from the gut of the tick to its salivary glands [3,7-9]. Piesman et al. identified bacteria present in the salivary glands of infected ticks before and during feedings [10]. It was shown by Lebet and Gern that 11% of nymphs, collected in the field, were systemically infected and by Leuba-Garcia et al. that 36% of adult ticks collected in Switzerland were systematically infected [11]. Although the exact time of tick attachment leading to transmission of disease has not been determined, the above suggests that every tick bite for any length of time cannot be ruled out and should be considered a potential point of infection. A limitation of the above studies is that attachment time relation to infection rate has not been tested in humans or in clinical trials and guidelines have been based purely from mice studies.
Lyme Disease Stages of Infection and Related Symptoms
The doubling time of B. burgdorferi is slow at approximately 12 hours [12]. This can affect presentations of symptoms. Patients can be asymptomatic until later stages of the infection. Symptoms of LD can present in one of three stages (if left untreated): early localized disease (<30 days from bite), early disseminated disease (< 3 months after bite), and late disseminated disease ( >3 months after bite). The early localized disease stage is characterized by erythema migrans, fever, arthralgia and/or headache. The early disseminated stage is characterized by additional symptoms of lymphadenopathy, atrioventricular block, tachyarrhythmias, and/or motor or sensory radiculopathy. The late disseminated stage is characterized by oligoarticular arthritis, encephalopathy, and/or encephalomyelitis [13]. However, it is of note that this list is not exhaustive, and symptoms may not appear in tandem. Some other common symptoms are: sweats, chills, achiness, malaise, sleep disruption, and myalgia [14].
Challenges in Lyme Disease and Tick-borne Illness Testing
Not only does the slow doubling time of B.burgdorferi affect symptom presentation but it also affects the types of viable testing available for diagnosis. The culturing of B.burgdorferi is therefore difficult (taking 12 weeks to be seen) and lacks sensitivity [15,16]. In addition, the window of time of bacteremia in which PCR can be used to detect is quite short making it hard to use for diagnostic purposes. It is for these reasons that the longer lived antibody IgM and IgG reactions are used in serology detection testing in blood [17].
The two-test or two-tier approach (TTS) for active or previous infection of lyme disease was proposed in 1994, using a sensitive enzyme immunoassay (EIA) or immunofluorescent assay (IFA) followed by a Western immunoblot. Positive or equivocal EIA or IFA would be confirmed by a Western immunoblot and negative EIA or IFA meant no further testing was needed [18]. An EIA uses an antigen to measure the subject’s blood’s antibody (IgG and IgM) response to that antigen quantitatively. The Western immunoblot detects antibody response (IgM or IgG) to a set of select antigens (seen as the bands on testing) taken from cultured lysates . The antibody response is then determined by the intensity of the bands compared to the control. The presence of multiple certain bands indicates a positive test result. One drawback to the TTS and the later developed modified two-tiered approach (MTTT) is that there is a gap in time between infection and the body’s development of antibodies where the test has little sensitivity [18-20]. In such cases, clinicians should obtain an acute and convalescent-phase serologic analysis due to this decreased sensitivity in the first few weeks of LD. Another drawback being that IgG antibodies can last for years and the information given by these tests is limited to merely indicating exposure to B. burgdorferi and they cannot give any indication to new, past or reinfection [17,19].
Some other common alternative tests for LD include Immunoblots (line blots), PCRs (polymerase chain reaction tests) and cerebrospinal fluid (CSF) analysis. Some other alternative testing media for these tests include skin biopsy, urine, and joint fluid. Immunoblots solve some of the reproducibility problems in Western blots, where the intensity of bands is determined visually. Instead Immunoblots take purified or recombinant proteins of B. burgdorferi placed in certain areas of the blot and then analyzed with densitometry (uses difference in transmitted and incident light to determine band intensity) [21,22]. Unfortunately, Immunoblots share drawbacks with Western immunoblots, and are also largely dependent on the development of antibodies to B. burgdorferi and limited to indicating exposure. PCRs do have some utility in providing specific evidence of B. burgdorferi nucleic acids in various media. However, low sensitivity, short testing window (in blood) and risk of sample contamination restrict its utility [22,23]. CSF analysis, similar to PCR, has a low yield of results due to transiency of spirochetes in blood as a result of high spirochete tropism to tissues such as the joint, heart, meninges, etc. [23,24]. Although the tests listed have varying benefits they have their own drawbacks and are best used to supplement clinical diagnosis.
Erythema Migrans as a Diagnostic Indicator
The early diagnosis of LD relies heavily on clinical diagnosis due to limitations in testing. Therefore, it is imperative to recognize early LD symptoms. In particular, the absence (or assumed absence) of the most common symptom used as an indicator of Lyme disease, erythema migrans (EM), does not definitively rule out LD. Erythema migrans has been estimated to present in more than 70% of early cases. While 27.5% of cases had arthritis, and 1.5% had carditis. Neurologic manifestations were seen in 12.5% of cases [25]. This estimate of high EM incidence causes both patients and clinicians to rely heavily on the presentation of this rash for diagnosis and initiation of treatment.
Diagnostic difficulties as a result of variation in erythema migrans presentation
Even if a patient presents with a rash following a bite it may be difficult to identify EM. Their EM rash can be misidentified as another type of bite or skin disease. There are other variations of the presentation of the rash that clinicians might not be aware of such as: homogenous regions without central clearing, lesions with more intense or dusky central regions [26-28], or a vesiculobullous or hemorrhagic center [29]. This leads to over and/or underdiagnosis of EM. In a study by Schotthoefer et al., a dermatologist and family practitioner with experience in early LD reviewed lesions of 69 participants suspected of having early LD. Of the 69 lesions, 51% of the lesions were identified as EM, 10% as possible early EM, 23% as tick bite reactions and 16% as non-EM reactions. During the initial review the reviewers agreed on the interpretation of 42 of the 69 lesions. The other 27 lesions had to be reevaluated due to disagreement in diagnosis. Of the 35 (51%) EM lesions, only 2 (6%) had a ring-within-a-ring or a bulls-eye pattern. The rash can present in a few days up to 30 days from the initial tick bite. Patients on average in the study reported onset of their EM rash 7.5 days after their initial bite [30].
Erythema migrans in CLD and PTLD
Identifying erythema migrans or other early Lyme disease symptoms is the best way to prevent complications and chronic or severe illness. However, it is of note that patients that identified as having chronic Lyme disease (CLD) or alternatively called post-treatment Lyme disease (PTLD), only remembered an erythema migrans rash approximately 33% of the time and remembered having a tick bite approximately 42% of the time [31]. If EM is not identified, never develops or the patient presents before development of the rash, it is important to factor in the area in which the patient frequents (whether endemic to ticks or not), if it is peak tick season [30], common LD symptoms such as fatigue, headache, fever, arthralgia, and if the patient owns a pet.
Monotherapy Antibiotics versus Combination Therapy Antibiotics For Persistent Lyme Disease
Although on average 68% patients with CLD report having been diagnosed late untreated LD, an average of 23% report an early LD diagnosis [31]. Among patients that have been treated acutely, the failure of treatment has been reported from 10 to 35% [31-35]. CLD patients with high treatment response (and therefore better outcomes) shared the following characteristics: use of antibiotics or a combination of antibiotics and alternative treatments, longer duration of treatment, and supervision by a clinician who focuses on treatment of tick-borne diseases [31].
The case against long-term use of (monotherapy) antibiotics
The Infectious Disease Society of America (IDSA) guidelines updated in 2021 recommended single drug therapy with oral doxycycline, amoxicillin, cefuroxime axetil, azithromycin or IV ceftriaxone, cefotaxime, penicillin G for 14 up to 28 day courses depending on symptom manifestation. In cases of persistent Lyme, IV ceftriaxone for 2-4 weeks was suggested over a second course of oral antibiotics [36]. A 2016 study by Berende et al. looked at the effects of longer term single oral antibiotic therapy following a 2-week course of IV ceftriaxone on Lyme patients with persistent symptoms. There were 3 study groups: a group receiving a 12-week oral course of doxycycline, a group receiving a 12-week course of clarithromycin and hydroxychloroquine and a placebo group. At the end of the study, the health-related quality of life score of the 3 groups were not significantly different and Berende et al. concluded no increase in patient health-related quality of life following longer duration doxycycline or clarithromycin-hydroxychloroquine [37].
A 2014 study looking at the effect of FDA approved drugs on B.burgdorferi persisters demonstrated that amoxicillin and doxycycline were indeed effective in killing log-phase (growth phase) bacteria but had little activity against stationary phase (persister phase) bacteria in vitro [38]. Of the drugs in the FDA-approved library 165 had higher activity against stationary phase B.burgdorferi than doxycycline and amoxicillin. Among the most active were: daptomycin, clofazimine, carbomycin, sulfamethoxazole and some cephalosporin antibiotics. Notably, daptomycin and clofazimine which had the highest activity against persister bacteria had little activity against growing bacteria [38].
Evidence of persistent Borrelia burgdorferi following conventional treatment
Although long-term single antibiotics have diminishing returns past 2 weeks, single antibiotics have been shown to be ineffective in persister or stationary forms of B. burgdorferi. Doxycycline and amoxicillin can be very effective against the replicating spirochetal form of B. burgdorferi but ineffective/less effective in other forms of B. burgdorferi [38] such as the spheroplast and cystic or round-body forms. Studies in animals such as primates (nonhuman), dogs and mice showed persistence of B. burgdorferi spirochetes after doxycycline or ceftriaxone treatment [39-42]. A study in 2019 examined sections of the brain, heart, kidney and liver of a 53-year old patient with a well-documented 6-year history of persistent Lyme disease treatment. The patient had no history of tick attachment or erythema migrans. The patient had lived in northern Westchester County, New York. She had an extensive history of IV cefotaxime, ceftriaxone, and oral minocycline courses (all at separate times). IHC experiments on 50 sections of each of the four organs (brain, heart, kidney, liver) demonstrated evidence of B. burgdorferi-positive spirochetal aggregates and clusters as well as biofilm-like aggregates in all four organs. The biofilms from all four organs were then confirmed to be positive for B. burgdoferi DNA using FISH [43].
The case for use of combination antibiotic therapy
Long-term single/non-combination antibiotic therapy has been shown to be ineffective for CLD. Unlike other persistent infections such as M. tuberculosis and Brucella melitensis, B.burgdorferi infections do not have established combination therapy treatments [44]. Since tationary phase cultures of B.burgdorferi contain varied populations of growing and non-growing bacteria with different morphologies, a second study by Feng et al. in 2015, examined the effectiveness of combination antibiotic therapy for B.burgdorferi persisters. First, Feng et al. assessed the percentage of different morphological forms of B.burgdorferi at various ages. They found the log-phase (3 days old) culture consisted of 96% spirochetal form, 4% round body form and no microcolony form. The stationary-phase (7 days old) culture consisted of 38% spirochetal form, 23% round body form, and 39% microcolony form. A 10 day culture in stationary form consisted of 20% spirochetal form, 26% round body form and 64% microcolony form [45]. Then, the antibiotic susceptibility of each morphological form of B.burgdorferi was determined. The different variant forms had differing susceptibilities. The older the culture was, the less it was susceptible and more tolerant the culture was to antibiotics (in monotherapy). The microcolony form was the most resistant to antibiotics, even to daptomycin and cefoperazone. The study concluded that a three drug combination of daptomycin, doxycycline and cefoperazone was able to eradicate even the most resistant microcolony form of B.burgdorferi [45]. A following study identified a second three drug combination of daunomycin or daptomycin with doxycycline and cefuroxime could also completely eradicate the most persistent form of B.burgdorferi.The study concluded that drug combinations addressing the different morphological forms of B.burgdorferi would best eradicate any persistence of the bacteria [46].
A 2023 study in mice compared the efficacy of drug monotherapy versus two to three drug combination therapy by testing the presence of B.burgdorferi DNA following drug therapy. Viable spirochetes were confirmed in all the monotherapy drug groups. In 8 out of 11 of the combination drug groups, viable spirochetes were not detected. The study showed the combinations of ceftriaxone + doxycycline, cefotaxime + doxycycline, Dapsone in combinations with rifampicin, clofazimine, or rifampicin + clofazimine were effective at eliminating viable B.burgdorferi spirochetes [44].
Citations available upon request.
About Ronald Stram, M.D.Related Blog Posts

Lyme Myth: Everyone with Lyme or a Tickborne Illness Needs to Avoid all Gluten and Dairy
Truth/Fact: Gluten sensitivities are extremely common, in part due to the pesticides used on conventionally grown wheat (called glyphosate or Roundup). This is also found on GMO crops such as corn and soy and can irritate the gut lining, weakening our immune system and having other impacts on our overall wellness. While many common food sensitivity tests are ineffective, an elimination diet along…Read the Post

Myth: A tick head needs to be removed completely if broken off in or under the skin.
According to the American Academy of Pediatrics 2020 and the Tick Bite, Schmitt Pediatric Guidelines 2020, it is recommended NOT to dig out the head of a tick if the tick breaks upon removal. It is felt that more trauma to the surrounding skin can lead to a higher risk of infection or cellulitis of the area. Leaving the tick head in does not increase the risk of contracting a…Read the Post
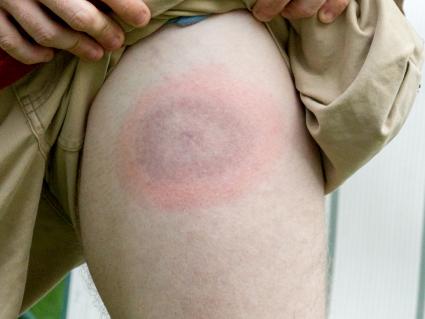
Myth: Bullseye Rash is Always Present in Lyme Disease
Many people believe that the bullseye rash, also known as erythema migrans (EM), is necessary for a Lyme disease diagnosis. While a bullseye rash is indeed clinically diagnostic, its absence cannot rule out either acute or chronic Lyme disease. However, a non-bullseye rash is more common...Read the Post

An Interview with Dr. Stram: How Effective are the Current Conventional Treatments for Lyme Disease?
In this interview, we asked the founder of the Stram Center, Dr. Ronald Stram, how effective current antibiotic protocols are in regard to Lyme disease treatment. Dr. Stram has devoted his career to those suffering from chronic illnesses, specifically tick-borne diseases. Since 2003, the Stram Center has served nearly 10,000 patients suffering from Lyme disease all across the globe. Dr. Stram…Read the Post
Related Services

Lyme Disease
Lyme Disease Diagnosis and Treatment in Delmar NY and Burlington VT offices At the Stram Center we vow to continue our education on Lyme Disease research, stay up to date on the most effective testing and all the safe available therapies. Moreover, our years of experience in treating patients according to the whole person-integrative medicine approach allows us the most effective way to care…Lyme Disease

Tests We Use
The Stram Center for Integrative Medicine utilizes the most advanced and comprehensive testing available to assess root cause of illness for our established patients.A wide range of detailed and extensive diagnostic tests are accessible by the Stram Center and include highly sensitive and accurate testing . Aside from conventional medical testing, such as Complete Blood Counts,…Tests We Use